Insights into the electronic properties and reactivity of graphene-like BC3 supported metal catalysts†
Abstract
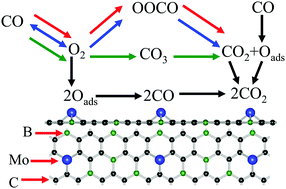
Graphene-like BC3 monolayer is a new two-dimensional nanomaterial with many unique properties, but is still largely unknown. First, we investigated the adsorption behaviors for different metal adatoms (Mo, Pd, Pt and Au) absorbed on pristine and defective BC3 (pri- and D-BC3) using first-principles calculations. It was found that the small barriers of the metal adatoms are more likely to diffuse on the pri-BC3 sheet; yet, the metal atoms doped BC3 systems (MD-BC3) exhibit high stability. Second, as O2 molecule is adsorbed more stably than CO, it regulates the electronic structures and magnetic properties of the MD-BC3 systems. Moreover, the possible reaction processes for CO oxidation on the Mo-BC3 substrate were systematically investigated using the Eley–Rideal (ER) and Langmuir–Hinshelwood (LH) mechanisms. For the ER mechanism, the preadsorbed O2 reacts with CO to form CO3 or the OOCO complex with smaller energy barrier (<0.30 eV) than that of the LH reaction. In comparison, the dissociative adsorption of O2 on the Mo-BC3 sheet as the starting step is an energetically more favorable process and the generated Oads atoms easily interact with two CO molecules via the ER reactions. This study provides insights into the physical properties and chemical reactivity of BC3-based nanomaterials.


 Please wait while we load your content...
Please wait while we load your content...