ESIPT-capable 2,6-di(1H-imidazol-2-yl)phenols with very strong fluorescent sensing signals towards Cr(iii), Zn(ii), and Cd(ii): molecular variation effects on turn-on efficiency†
Abstract
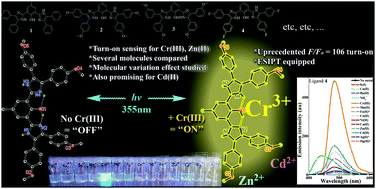
A series of structurally and electronically varied 2,6-di(1H-imidazol-2-yl)phenols that are ESIPT-capable (1–12) as well as the ESIPT-incapable 4,5-diphenyl-2-(3-(4,5-diphenyl-1H-imidazol-2-yl)phenyl)-1H-imidazole (13) were designed and comparatively studied for their molecular effects on their sensitivity and selectivity characteristics as fluorescent chemosensors of Cr3+, Zn2+, and Cd2+. Their single-crystal structures revealed a desired chain of intramolecular hydrogen bonding (ligand 12) as well as possible coordination modes. Probes 1–4 demonstrated very high turn-on sensitivity and selectivity as double fluorescent sensors for Cr3+ and Zn2+ at their different emission wavelengths (blue-shifted in the case of Zn2+). A remarkable 106-fold emission turn-on by Cr3+ was recorded for molecule 2, which, to the best of our knowledge, is an unheard of magnitude for Cr3+ sensitivity. The results suggested that a Cd2+ sensor could be developed by further derivatization of these probes. The possession of symmetrical substitution on both imidazole rings, multiple active protons involved in hydrogen intramolecular bonding relays, and the consequent ESIPT capability of the studied molecules were found to be very beneficial to their successful outcomes as high fluorescent turn-on chemosensors. Modification of the sensor properties, such as sensitivity and selectivity, was achieved through substituent manipulations at certain peripheral positions. Thus, deliberate molecular derivatization was found to be a tool for manipulating the interference and selectivity profiles. The Job plot, single-crystal results, and sustained large Stoke's shift in the presence of Cr3+ suggest a one-pocket N^O coordination in a 1 : 1 stoichiometry rather than a binuclear N^O^N two-pocket coordination. Quantum mechanical calculations on the model structures suggested that the successful turn-on results may be associated with the coplanarity settings of the imidazole and phenol rings.
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...