Xerogel modified diatomaceous earth microparticles for controlled drug release studies†
Abstract
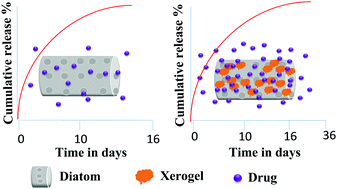
Naturally available diatomaceous earth (DE) microparticles are ideal candidates for drug delivery due to their excellent features like biocompatibility, non-toxicity, porosity, high surface area and ease of surface modification. On the other hand, they have some limitations, especially in drug delivery applications, such as poor drug loading capacity and a very high initial burst release. In order to address these drawbacks, we have surface modified the diatoms with silica xerogel, which forms a novel hybrid material. The modification process was carried out by a facile sol–gel method, the silica xerogel decorated DE microparticles were extensively characterized by SEM, BET, ATR-IR spectroscopy and XRD in order to confirm the covalent linkage of the new material on the surface of the DE microparticles. The prepared hybrid material DE-XER (xerogel) acts as a pH-sensitive micro drug carrier for diclofenac sodium (DS) drug. The results indicate that surface modification plays a critical role, enhancing the drug loading capacity in comparison with neat DE microparticles, achieving effective controlled release. Furthermore, the obtained drug release data were fitted to the zero order model to understand the drug release mechanism.


 Please wait while we load your content...
Please wait while we load your content...