Negatively charged boron nitride nanosheets as a potential metal-free electrocatalyst for the oxygen reduction reaction: a computational study†
Abstract
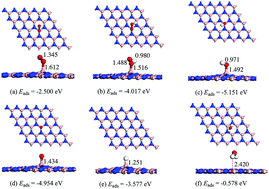
The high cost and poor stability in electrochemical environments of the Pt-based catalysts conventionally employed in the oxygen reduction reaction (ORR) have hindered the large-scale commercialization of fuel cells. Therefore, high-activity, low-cost, and stable metal-free catalysts to replace Pt-based catalysts are desirable. Herein, by means of density functional theory (DFT) computations, we explored the possibility of employing negatively charged boron nitride (BN) nanosheets, which are easy to fabricate, as an ORR electrocatalyst. The results show that O2 can be strongly chemisorbed onto the charged BN nanosheet, which is an essential step in initiating the reaction sequence. The calculated activation energies and reaction energies of each ORR step indicated that the ORR can easily occur via a direct four-electron pathway to form two H2O molecules, in which the rate-determining step was OOH* + H+ + e− → 2OH*, with an activation energy of 0.561 eV, and the computed limiting potential was about 0.676 V. These results demonstrate that negatively charged BN nanosheet a promising metal-free ORR electrocatalyst with high efficiency.


 Please wait while we load your content...
Please wait while we load your content...