Enhanced Li ion conductivity in Ge-doped Li0.33La0.56TiO3 perovskite solid electrolytes for all-solid-state Li-ion batteries
Abstract
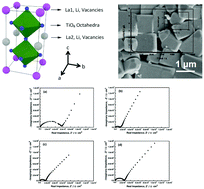
Ge-Doped Li0.33La0.56TiO3 (Ge-LLTO) perovskites are synthesized with various GeO2 contents by solid-state reactions as Li-ion conductive electrolytes for rechargeable all-solid-state Li batteries. After the addition of GeO2, the sinterability and Li ion conductivity are enhanced for the perovskite solid electrolyte. The Li0.43La0.56Ti0.95Ge0.05O3 electrolytes exhibit the highest sinterability and Li ion conductivity among the as-prepared Li0.33+2xLa0.56Ti1−xGexO3 electrolytes. The improvement of Li ion conductivity might be attributed to the improvement of the densification and structural integrity of the LLTO solid electrolyte. The lithium transference number (tLi+) of Ge-doped LLTO is close to 1. Moreover, the Ge-LLTO electrolytes are stable in the potential range of 0–10 V vs. Li/Li+.


 Please wait while we load your content...
Please wait while we load your content...