Aerobic oxidative esterification and thioesterification of aldehydes using dibromoisocyanuric acid under mild conditions: no metal catalysts required†
Abstract
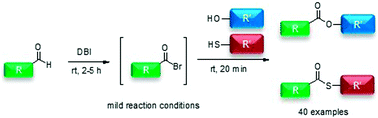
A practical direct method for the direct preparation of esters and thioesters from aldehydes is described. Esters and thioesters were synthesized by oxidative esterification and thioesterification via in situ generated acyl bromide intermediates, which were used to react with various alcohols and thiols. The esterification and thioesterification were readily performed in the presence of dibromoisocyanuric acid in dichloromethane, without any metal catalysts and under mild conditions. By using this reaction protocol, various esters and thioesters were prepared in high yields. This effective method offers a promising approach for the facile esterification and thioesterification of aldehydes.


 Please wait while we load your content...
Please wait while we load your content...