Bio-based triacetic acid lactone in the synthesis of azaheterocycles via a ring-opening transformation†
Abstract
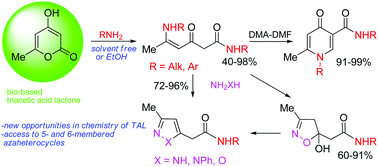
In the present article a new way of converting bio-based triacetic acid lactone (TAL) into azaheterocyclic compounds, such as 4-pyridones, pyrazoles, isoxazolines and isoxazoles, has been found. The main strategy involves the transformation of TAL into reactive and multifunctional polycarbonyl intermediates serving as C-6 building blocks for the construction of organic compounds. TAL undergoes a ring opening transformation with aliphatic and aromatic amines, including bioavailable amines, under solvent free conditions or in EtOH to give carbamoylated enaminones (40–98%). These polyfunctional compounds have been converted into biologically important pyridone-3-carboxamides (91–99%) with dimethylformamide dimethyl acetal and also react regioselectively with hydrazines and hydroxylamine to form pyrazolyl- (72–96%) and (isoxazolinyl)acetoamides (60–91%). The conversion of TAL into hetarylacetic acid amides can be performed as one-pot reactions without isolation of polycarbonyl intermediates.


 Please wait while we load your content...
Please wait while we load your content...