Controlling the polymerization of coniferyl alcohol with cyclodextrins†
Abstract
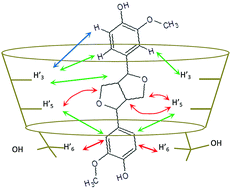
The mono-electronic oxidation of coniferyl alcohol leads to the formation of phenoxy radicals and ultimately to the synthesis of dimeric lignan and neo-lignan products. Coniferyl alcohol and dimeric products are all potential guests for cyclodextrins (CDs) to form noncovalent host–guest inclusion complexes. Herein, the influence of CDs with different cavity volumes (i.e., α, β or CD) on the laccase-driven oxidation of coniferyl alcohol is studied. We clearly show that βCD interacts with the dimeric products and selectively prevents their further oxidation by the enzyme. Moreover, amongst the three dimeric products generated, the system containing a laccase and βCD allows the selective enrichment of pinoresinol; this behaviour is similar to that of plant dirigent proteins.


 Please wait while we load your content...
Please wait while we load your content...