The effects of bromine atoms on the photophysical and photochemical properties of 3-cinnamoylcoumarin derivatives†
Abstract
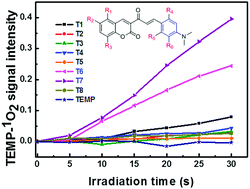
Eight coumarin derivatives (T1–T8) with or without modification using one or two bromine atoms were synthesized through the aldol condensation reaction. Their photophysical, photochemical and electrochemical properties were investigated. It was shown that the position and the number of bromine atoms had a great effect on the properties of these compounds. When bromines were linked onto the coumarin ring (mainly in the LUMO) directly, the absorption and emission spectra of T2–T5 all displayed red shifts but no obvious difference was found in their photochemical properties compared to T1 without modification. In contrast, T6 and T7 with one bromine linked onto the right benzene ring (mainly in the HOMO) exhibited enhanced capability of generating singlet oxygen and decreased photostability caused by the transformation between their two conformers, while T8 with two bromines on the benzene ring was photobleached rapidly due to a great difference in the torsional angle between the substituted phenyl ring and the linkage part in the ground state and the excited state.


 Please wait while we load your content...
Please wait while we load your content...