A highly sensitive pyridine-dicarbohydrazide based chemosensor for colorimetric recognition of Cu2+, AMP2−, F− and AcO− ions†
Abstract
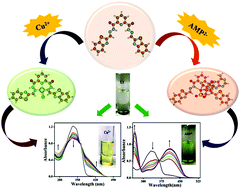
A new pyridine-dicarbohydrazide based colorimetric chemosensor L1 is designed and synthesized for the detection of anions and cations. The chemosensor allows the ‘naked-eye’ recognition of toxic cation Cu2+ and biologically relevant anions AMP2−, F− and AcO− in DMSO/H2O (8/2, v/v) solution with a fast response time (∼5 s). The selectivity, sensitivity and binding stoichiometry of L1 for the aforementioned ions are investigated using colorimetric, UV-Vis spectroscopic and 1H NMR studies. The binding mode is analyzed by UV-Vis and 1H NMR studies, which are supported by density functional theory (DFT) and time-dependent density functional theory (TDDFT) calculations. The optimized geometries indicate that the carbohydrazide moiety provides the binding site for the cation and anions. The HOMO–LUMO energy gap for the complex of AMP2− with L1 is least among all the complexes, possibly due to the charge-transfer effect. The detection limits of L1 for Cu2+ and AMP2− were determined to be 0.12 μM and 0.08 μM, respectively. Furthermore, the potential application of the sensor for the detection of fluoride ion in commercially available toothpaste was demonstrated successfully.


 Please wait while we load your content...
Please wait while we load your content...