The study of the influence of Mg(ii) and Ca(ii) ions on caffeic acid autoxidation in weakly alkaline aqueous solution using MCR-ALS analysis of spectrophotometric data†
Abstract
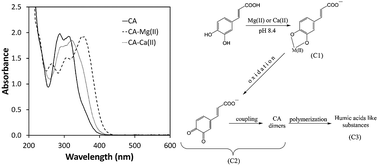
UV-Visible spectrophotometry was used to study the influence of Mg(II) and Ca(II) ions on caffeic acid (CA) autoxidation in weakly alkaline aqueous solution. While the autoxidation rate was very slow in the absence of metal ions, both Mg(II) and Ca(II) ions greatly enhanced the autoxidation rate. Determination of the spectral and concentration profiles of the individual components involved in the CA autoxidation processes in the presence of metal ions was performed using multivariate curve resolution-alternating least squares (MCR-ALS) analysis of spectrophotometric data. MCR-ALS analysis supported by the quantum chemical calculations of electronic absorption spectra for possible initial reaction products showed that CA autoxidation in the presence of Mg(II) and Ca(II) ions proceeds by the instant formation of CA–metal complexes followed by the almost simultaneous formation of CA quinone anions and various CA dimers and the final transformation into polymeric, humic acid-like, products. Also, the MCR-ALS analysis revealed that the effect of Mg(II) ions was more pronounced at the initial stage of autoxidation. The differences in the influence of Mg(II) and Ca(II) ions on the CA autoxidation may be attributed to the stronger metal ion–ligand interaction for Mg(II) ions caused by their larger ionic potential in comparison to Ca(II) ions. The results of this study may be important for better understanding of the interactions of Mg(II) and Ca(II) ions with natural phenolic food antioxidants and thus significant for understanding their activities in real systems and optimization of conditions for the processing and/or storage of certain types of foods.


 Please wait while we load your content...
Please wait while we load your content...