A quinoline-based compound for explosive 2,4,6-trinitrophenol sensing: experimental and DFT-D3 studies†
Abstract
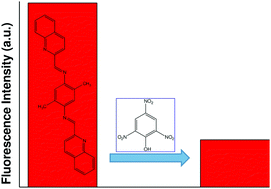
A quinoline-based Schiff base compound, 2,5-dimethylbis(quinolin-2-ylmethylene)benzene-1,4-diamine (DQB), has been found to be a chemosensor for 2,4,6-trinitrophenol (TNP). DQB has been synthesized by condensation between two equivalents of quinoline-4-carboxaldehyde and one equivalent of 2,5-dimethyl-1,4-diaminobenzene in methanol under ambient reaction conditions and characterized by using standard spectroscopic methods. It shows an emission band at 440 nm with high intensity (λex = 390 nm). The emission intensity is quenched severely in the presence of TNP while other nitroaromatic compounds fail to reduce the intensity, signifying high selectivity towards TNP. The fluorescence quenching mechanism can be explained by theoretical calculations as an RET-ICT based pathway.


 Please wait while we load your content...
Please wait while we load your content...