Enantiomeric separation and simulation study of eight anticholinergic drugs on an immobilized polysaccharide-based chiral stationary phase by HPLC
Abstract
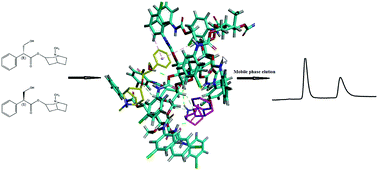
The enantiomeric separation of eight anticholinergic drugs was first systematically examined on a derivative polysaccharide chiral stationary phase (CSP), i.e. Chiralpak ID in the normal phase mode. Except for scopolamine hydrobromide and benzhexol hydrochloride, the other six analytes including atropine sulfate, phencynonate, dipivefrine hydrochloride, tropicamide, homatropine methylbromide and oxybutynin were either completely or partially separated under the optimized mobile phase conditions with resolutions of 3.98, 2.52, 2.02, 2.14, 1.80 and 0.41, respectively. The influences of organic modifier types and content, and base/acid additives on enantiomeric separation were evaluated and optimized. Furthermore, a simulation study was also used to explain the chiral recognition mechanisms of this class of drug enantiomers on Chiralpak ID for the first time. The modeling data were in agreement with the chromatographic results concerning enantioselectivity.


 Please wait while we load your content...
Please wait while we load your content...