Removal of anionic dyes from an aqueous solution by a magnetic cationic adsorbent modified with DMDAAC†
Abstract
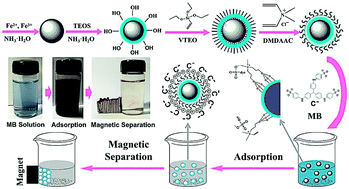
Herein, a novel magnetic adsorbent (Fe3O4@SiO2–VTEOS–DMDAAC) was synthesized by polymerizing the carbon–carbon double bonds of triethoxyvinylsilane (VTEO) and dimethyl diallyl ammonium chloride (DMDAAC) for the enhanced removal of anionic dyes from aqueous solutions. Characterization of the as-prepared magnetic adsorbents was conducted by SEM, TEM, FTIR, XRD, and TGA. Methyl blue (MB) was selected as a model dye to investigate the removal performance. In the adsorption process, various parameters, such as adsorbent dosage, initial solution pH, initial dye concentration, and reaction time were studied and optimized. Under the optimal condition (11 mg adsorbent dosage, 3.0 pH, 19 mg L−1 MB and 2 h adsorption time), a maximum adsorption capacity (qm) for MB (109.89 mg g−1) was achieved. The quaternary ammonium groups existing on the surface of magnetic adsorbents could promote the adsorption of MB via electrostatic attractions. Kinetics study revealed that the adsorption processes follow the pseudo-second-order model. The equilibrium data matched well with the Freundlich isotherm model. The as-prepared magnetic adsorbents could be effectively separated from the medium under an adscititious magnetic field after the loading of the adsorbate and could be easily regenerated. The magnetic adsorbents exhibited good reusability in three cycles of reuse. This magnetic adsorbent could be a promising candidate for the treatment of wastewater containing anionic dyes.


 Please wait while we load your content...
Please wait while we load your content...