One step synthesis of a fused four-ring heterocycle†
Abstract
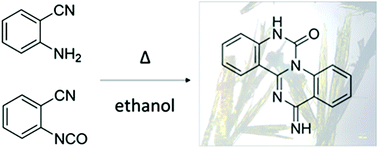
A wide range of natural biomolecules and pharmaceuticals are nitrogen rich heterocycles. Though preparation of fused multi-ring systems typically requires multiple synthetic steps, 13-imino-7,13-dihydro-6H-quinazolino[3,4-a]quinazolin-6-one (IDQQ) was serendipitously obtained in one step from commercially available phenylic precursors. Single crystal X-ray diffraction, 1H-NMR and density functional theory calculations (DFT/B3LYP/6-31G(d,p)) were used in combination to unambiguously assign the major tautomer present in the monoclinic crystals. IDQQ appears to be the product of a thermodynamically-driven solution rearrangement of a key intermediate, 1,3-bis(o-cyanophenyl)urea. This type of cyclization may be an efficient method for the formation of other related heterocycles.


 Please wait while we load your content...
Please wait while we load your content...
