The role of Cr, Mo and W in the electronic delocalization and the metal–ring interaction in metallocene complexes
Abstract
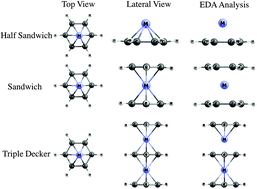
Metal influence over triple-decker, sandwich-like and pyramidal structured benzenes was studied by means of Energy Decomposition Analysis (Morokuma–Ziegler), combined with Extended Transition State Natural Orbitals for Chemical Valence, finding that metal–ring bonding was a covalent contribution of about 60% due to the bonding interaction between dxz and dyz, dx2−y2 and dxy orbitals with pz orbitals, respectively, adapted by symmetry in the ring, to form π and δ bonding interactions. Finally, an important amount of electron density between the ring and the metal was found. This has a key role in the electron delocalization in this zone. This electronic delocalization was analysed via Induced Magnetic Field and Nucleus-Independent Chemical Shift calculations, finding a pattern between metal atomic radii and shielding tensor. Furthermore, similar behaviour for Mo and W, in the enhancement of the diatropic magnetic response, was displayed while Cr had a slightly lower diatropic character.


 Please wait while we load your content...
Please wait while we load your content...