Alizarin red S–TiO2-catalyzed cascade C(sp3)–H to C(sp2)–H bond formation/cyclization reactions toward tetrahydroquinoline derivatives under visible light irradiation†
Abstract
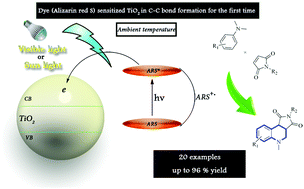
A very low amount of organic dye (Alizarin red S) sensitized TiO2 and it was successfully used to catalyze cascade C(sp3)–H to C(sp2)–H bond formation/cyclization reactions under visible light irradiation. The modified TiO2 photocatalyst efficiently, for the first time, advanced [4+2] cyclization of N,N-dimethylanilines and maleimides to the corresponding tetrahydroquinolines in air atmosphere. The reaction proceeds through α-amino radicals without additional oxidant at ambient temperature to afford products in good to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...