Room temperature ionic liquids versus organic solvents as lithium–oxygen battery electrolytes
Abstract
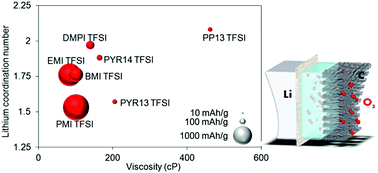
Imidazolium, pyrrolidinium and piperidinium room temperature ionic liquids (RTILs) are characterized to evaluate their thermal stability, ionic conductivity, viscosity, potential window and lithium solvation, which are key parameters for lithium–oxygen (Li–O2) battery electrolytes. The electrochemical tests of these RTILs are also carried out in Li–O2 cells and show reasonable values of specific capacity, up to 1659 mA h per gram of carbon in the case of 1-propyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (PMI TFSI) with a molar fraction of 0.2 of LiTFSI. RTILs are compared with conventional organic electrolytes like ethylene carbonate (EC)/diethyl carbonate (DEC) solution or tetraethylene glycol dimethyl ether (TEGDME), which enables us to provide a further insight into ways of improving the electrolyte properties of Li–O2 batteries. In particular, a viscosity lower than 100 cP combined with a lithium coordination number lower than 1.5 tends to enhance the battery performance.


 Please wait while we load your content...
Please wait while we load your content...