Syntheses, structures and anti-tumor activity of four organotin(iv) dicarboxylates based on (1,3,4-thiadiazole-2,5-diyldithio)diacetic acid†
Abstract
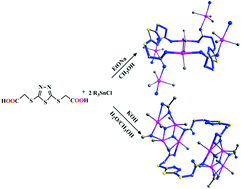
Four novel organotin complexes, [(Me3Sn)2(O2CCH2S)2C2N2S]n (1), [(Me2Sn)2(O2CCH2S)2C2N2S(μ3-O)]n (2), [(n-Bu3Sn)2(O2CCH2S)2C2N2S]n (3) and [(n-Bu2Sn)(O2CCH2S)2C2N2S]n (4), derived from flexible (1,3,4-thiadiazole-2,5-diyldithio)diacetic acid (H2tzda), have been synthesized and characterized by elemental analysis, FT-IR, NMR and X-ray crystallography. The structural analysis reveals that complexes 1 and 2 display a 1D pipeline-like and 1D ladder chain structure, respectively. Complex 1 contains interconnected 26-membered macrocycles, while complex 2 is made up of the typical ladder tetraorganodistannoxane unit containing 26-membered macrocycles. In addition, the molecules of complexes 1 and 2 are further linked through intermolecular C–H⋯O interactions into a 3D and 2D infinite supramolecular structure, respectively. Complex 3 shows a 2D corrugated polymeric structure constructed by 34-membered macrocycles. Complex 4 adopts a 1D infinite chain structure. Furthermore, in vitro cytotoxicity investigations of complexes 1, 3 and 4 were conducted. Importantly, further cytotoxic assessments of complex 3 against human hepatocellular carcinoma cells (HepG-2) demonstrate that complex 3 could induce an apoptotic retardation effect on proliferation via possible mitochondrial-dependent cross-talk between various signaling proteins and the intracellular p53-Bax-caspases approach should be the major pathway of apoptosis.


 Please wait while we load your content...
Please wait while we load your content...