Synthesis of a class of binaphthyl monophosphine ligands with a naphthofuran skeleton and their applications in Suzuki–Miyaura coupling reactions†
Abstract
A series of new monophosphine ligands containing a naphthofuran skeleton have been prepared, characterized and evaluated in palladium-catalyzed Suzuki–Miyaura coupling reactions. Using Pd2(dba)3–L6 as the catalyst the reactions were performed with high catalytic activity under mild reaction conditions and the desired coupling products were obtained in good to excellent yields. The new catalyst system also showed broad substrate adaptability and practicality for gram-scale production.
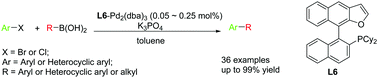


 Please wait while we load your content...
Please wait while we load your content...