Sn-Doped defect pyrochlore oxide KNbWO6·H2O microcrystals and their photocatalytic reduction of CO2†
Abstract
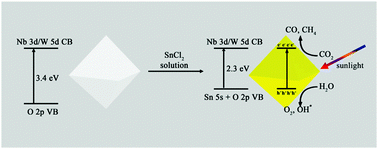
It is highly desirable to develop new semiconductors for the photocatalytic reduction of CO2 to CH4 and CO to solve greenhouse effect and energy issues. Defect pyrochlore oxides have a flexible composition and the electron/hole mobility can be manipulated by introducing foreign cations into the structure. Through Sn doping into the defect pyrochlore oxide KNbWO6·H2O, we successfully obtained a 2-times higher photocatalytic activity for converting CO2 to CO, CH4 and O2 compared to the original KNbWO6·H2O. According to the data from UV-vis, photoluminescence, and hydroxyl radical amount-related fluorescence spectra, and CO2 adsorption, the improved photocatalytic activity can be attributed to the extended visible light response, the enhanced charge separation and the improved CO2 adsorption due to Sn doping. Our results provide a new strategy for developing high-performance photocatalysts.


 Please wait while we load your content...
Please wait while we load your content...