DMAP-catalyzed alkylation of isatin N,N′-cyclic azomethine imine 1,3-dipoles with Morita–Baylis–Hillman carbonates†
Abstract
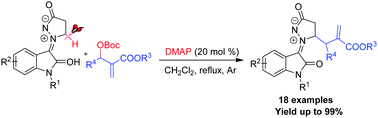
Azomethine imines are valuable substrates in catalysis, and can be precursors to complex 3-substituted oxindoles. An efficient and convenient synthetic approach to azomethine imines is developed via DMAP-catalyzed direct alkylation at the α-position of the cyclic amine of the new isatin N,N′-cyclic azomethine imines with Morita–Baylis–Hillman carbonates. A variety of α-alkylated-isatin N,N′-cyclic azomethine imine 1,3-dipoles were obtained in excellent yields (up to 99%) under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...