Taking the next step toward inert Mn2+ complexes of open-chain ligands: the case of the rigid PhDTA ligand†‡
Abstract
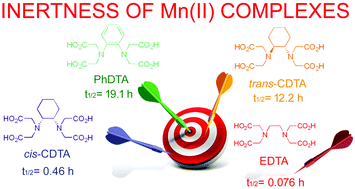
In line with our research to find inert Mn(II) complexes as contrast agents for magnetic resonance imaging, we have studied the aromatic-ring rigidified EDTA-analogue o-phenylenediamine-N,N,N′,N′-tetraacetic acid (PhDTA). The protonation constants (KHi) of PhDTA and stability constants of complexes formed between this open-chain ligand and several different biogenic metal ions (Ca2+, Mg2+, Zn2+, Cu2+, Mn2+) have been determined in 0.15 M NaCl at 25 °C and compared with the values reported in the literature previously. The protonation constants are lower than those of the corresponding cis- and trans-CDTA complexes, which might be attributed to the electron withdrawing effect of the phenylene group. The lower total basicity of the ligand leads to lower stability constants for all the examined metal complexes. On the contrary, we have found that the conditional stability constants of [Mn(PhDTA)]2− and [Mn(trans-CDTA)]2− are approximately the same, as both complexes are completely formed at pH 5 and their pM values are also comparable. The relaxivity of [Mn(PhDTA)]2− is nearly identical (r1 = 3.72 mM−1 s−1) to that determined previously for the [Mn(trans-CDTA)]2− complex (r1 = 3.62 mM−1 s−1), and its pH-dependence confirms the equilibrium model used for the fitting of the titration data. The results of the kinetic studies of the metal exchange reactions reveal that the [Mn(PhDTA)]2− complex possesses a slightly better dissociation kinetics profile than that of [Mn(trans-CDTA)]2−, which has been tested in vivo recently (including human injections). The half-life of the dissociation of the complex near physiological pH at 25 °C is 19 hours. By using the rate constant calculated for the dissociation (pH = 7.4, cCu2+ = 10 μM) and the half-life of excretion (1.6 hour), the ratio of the dissociated complex is estimated to represent 8% of the injected dose. DFT studies reveal that the metal coordination environment of [Mn(PhDTA)]2− is very similar to that of [Mn(EDTA)]2−, both containing an inner-sphere water molecule. Cyclic voltammetry studies indicate that [Mn(PhDTA)]2− is slightly more resistant towards oxidation to the Mn3+ complex than the EDTA analogue.
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...