Facile synthesis of oxidized activated carbons for high-selectivity and low-enthalpy CO2 capture from flue gas†
Abstract
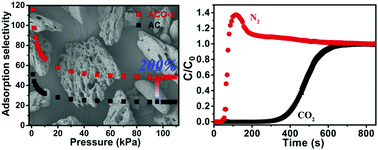
The prospect of a worsening climatic situation prompts us to develop energy-saving and cost-effective CO2 capture technologies. In this work, three oxidized activated carbons (ACO-n, n is 1–3) were prepared through a facile synthesis approach via oxidation in the presence of KMnO4 and concentrated H2SO4 for 0.5, 1.0 or 2.0 hours. Interestingly, these carbon materials ACO-n can exhibit high CO2 capacity with low adsorption enthalpy and the selectivity toward flue gas can be adjusted by altering the period of oxidation. Among the pristine activated carbon and ACO-n materials, ACO-2 can exhibit the highest CO2 capacity of 3.01 mmol g−1 under ambient conditions with an adsorption enthalpy of only 23.1 kJ mol−1, slightly larger than the CO2 vaporization enthalpy. Its selectivity of 48.5 is double the value of the pristine activated carbon. A column breakthrough experiment was conducted to evaluate the CO2 separation capability on ACO-2 toward a CO2/N2 (15 : 85 v/v) mixture under kinetic flow conditions, which suggests that the oxidized activated carbon made from sustainable sources is promising for CO2 capture.


 Please wait while we load your content...
Please wait while we load your content...