J-Aggregation induced emission enhancement of a thienyl substituted bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine (BOPHY) dye†
Abstract
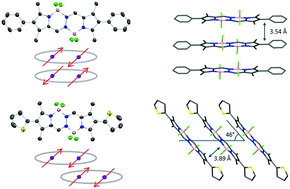
The synthesis and characterization of 2,7-diphenyl- and 2,7-dithienyl-1,3,6,8-tetramethyl bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine (BOPHY) derivatives 1 and 2 are described. The compounds were characterized by NMR (1H, 13C), X-ray single crystal diffraction analysis, UV/Vis, fluorescence spectroscopy and high-resolution mass spectrometry (HRMS). The photophysical properties were investigated in solvents of different polarities. Compound 1 exhibits slight solvent dependent spectroscopic properties, whereas compound 2 shows fluorescence quenching with an increase in the solvent polarity. Aggregation-induced emission enhancement (AIEE) is observed in compound 2, which is attributed to the formation of J-aggregates that result in narrow, red-shifted and enhanced emission. Modification of the β-substituents of BOPHY is responsible for the formation of these aggregates, which is definitively established by an X-ray diffraction study.
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...