Friedelane triterpenoids: transformations toward A-ring modifications including 2-homoderivatives†
Abstract
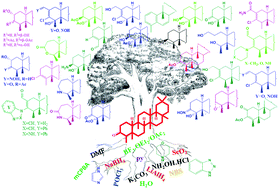
Friedelin and its derivatives, commonly known as friedelane triterpenoids, exhibit potential biological effects ranging from antimicrobial to anticancer to anti-HIV. To modify the A-ring of the pentacyclic triterpenoid, various transformative scopes have been utilized. Herein, some simple unprecedented transformative protocols have been accomplished towards furnishing 42 (25 new) A-ring modified pentacyclic friedelane triterpenoids. It is worth noting that the modifications include the all-new 2-homo derivatives. The one-pot BF3·OEt2-mediated oxidative transformation of friedelin to yield friedel-3-enol acetate as the major product was one of the key reactions. A group of isomeric A-ring modifications was produced on the basis of simple transformations on suitable friedelane-based molecules. The syntheses of the novel 2-homofriedelanes were envisioned from the transformative reactions of the designed triterpenoid 3-chlorofriedel-2-ene-2-carbaldehyde, which was isolated as the major product from the reaction of friedelin with the novel Vilsmeier–Haack reagent. New A-ring modified derivatives were also obtained due to further interesting transformations of 3-chlorofriedel-3-ene, isolated as side products from the same reaction. Again, considering the scope of the 3-chloro-2-enal moiety associated with the A-ring of the triterpenoid, some heterocycle-linked- (bonded to C3) 2-homofriedelane triterpenoids were synthesized. Various common reaction strategies were employed on suitable substrates to finally achieve a series of C2,C3-; C3,C4- and C2,C3,C4-functionalized as well as 2-homofriedelane triterpenoids with just one to four efficient steps.


 Please wait while we load your content...
Please wait while we load your content...