Triflic acid-catalyzed metal-free synthesis of (E)-2-cyanoacrylamides and 3-substituted azetidine-2,4-diones†
Abstract
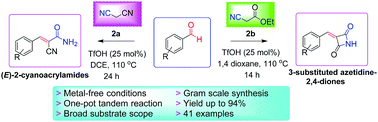
A TfOH-catalyzed highly efficient synthesis of biologically active (E)-2-cyanoacrylamides and 3-substituted azetidine-2,4-diones has been reported with 64–94% yields under metal-free conditions. The reaction proceeds through sequential Knoevenagel condensation/stereoselective in situ monohydration of nitrile or C–N cyclization protocol in one-pot. The attractive features of this tandem process are moderate reaction conditions, high atom economy, broad substrate scope, gram-scale reaction and ease of operation.


 Please wait while we load your content...
Please wait while we load your content...