Synthesis of thorium–ethanolamine nanocomposite by the co-precipitation method and its application for Cr(vi) removal†
Abstract
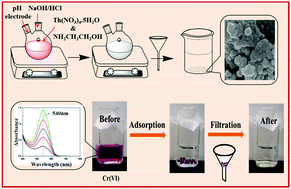
In the present work, a thorium–ethanolamine (Th–Eth) nanocomposite was synthesized by the co-precipitation method and used to investigate the removal of Cr(VI) from aqueous solution. The material was highly selective for Cr(VI) removal due to its high surface area (67 m2 g−1), low pore size (3.2 nm), small particle size (4.5 nm) and good ion exchange properties. The adsorption process obeyed pseudo-second-order kinetics and the adsorption data were fitted well to the Langmuir model with an R2 value of 0.995 with a maximum adsorption capacity of 109.89 mg g−1. The E (10.62 kJ mol−1) value indicated the chemical nature of the adsorption process. About 99.53% Cr(VI) was removed under the optimum conditions of a solution pH of 5, an adsorbent dose of 1.2 g L−1, a stirring rate of 200 rpm and an equilibrium time of 60 min with 10 mg L−1 initial Cr(VI) concentration in batch adsorption experiments. The material showed significant removal of Cr(VI) even in the presence of other anions like carbonate, bicarbonate, nitrate, phosphate, fluoride, chloride and sulfate. The material was desorbed with 0.5 M NaOH solution and regenerated materials still possess a high adsorption capacity up to five consecutive cycles. The current study showed that Th–Eth can be used as an effective recyclable material for Cr(VI) removal.


 Please wait while we load your content...
Please wait while we load your content...