Functionalization of fluorodinitroethylamino derivatives based on azole: a new family of insensitive energetic materials†
Abstract
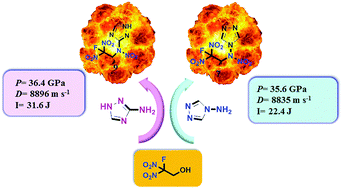
In this work, four energetic N-fluorodinitroethyl-substituted aminoazoles 6, 8, 10 and 11, as well as nitramine 7 and 9 (the N-nitration products of 6 and 8, respectively) are synthesized. All compounds are characterized by multinuclear NMR spectroscopy, IR, elemental analysis, as well as differential scanning calorimetry (DSC); what's more, the structures of compounds 6, 9 and 10 are confirmed by single crystal X-ray diffraction. In addition, the sensitivities of 6–10 are measured by standard impact and friction tests. The results indicate that the reported compounds are less sensitive than TNT. The detonation performance based on the calculated heat of formation and experimental densities illustrates that most of the compounds might be of interest for applications as insensitive energetic materials, especially for compounds 7 and 9 (D = 8835 and 8896 m s−1; P = 35.6 and 36.4 GPa).


 Please wait while we load your content...
Please wait while we load your content...