Solvatochromism of conjugated 4-N,N-dimethylaminophenyl-pyridinium donor–acceptor pairs†
Abstract
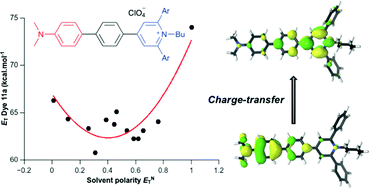
A series of solvatochromic dyes consisting of an N,N-dimethylaminophenyl donor group conjugated with a 1-butylpyridinium acceptor ring through a 1,4-phenylene spacer were synthesized by a three-step procedure. The procedure involved the synthesis of 4-bromophenylpyrylium salts, their conversion to N-butylpyridinium derivatives, and palladium-catalysed Suzuki–Miyaura coupling reactions. Their UV-vis spectroscopic behaviours, which were investigated in 13 solvents, were compared with the solvatochromism of an amino analogue of Reichardt's ET(30) betaine. All the synthesized dyes showed inverted solvatochromism, which was rationalized by theoretical calculations.


 Please wait while we load your content...
Please wait while we load your content...