Metal ion complexes of nucleoside phosphorothioates reflecting the ambivalent properties of lead(ii)
Abstract
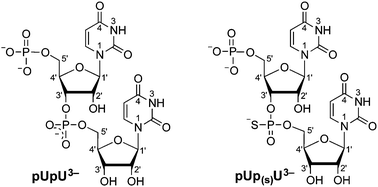
This Perspective outlines the coordinating properties of lead(II), to some extent in comparison with related metal ions like Ca2+, Zn2+ or Cd2+. It is worth noting that the affinity of Pb2+ towards phosphate residues corresponds to that of Cu2+. Furthermore, the binding tendency of Pb2+ towards thiophosphate groups as present in methyl thiophosphate (MeOPS2−) or uridine 5′-O-thiomonophosphate (UMPS2−) is compared with that of the parent ligands, that is, methyl phosphate (CH3OPO32−) and uridine 5′-monophosphate (UMP2−). The replacement of an O by a S atom makes the monoprotonated thiophosphate group considerably more acidic [compared to ROP(O)2−(OH)], but at the same time its affinity for Pb2+ increases tremendously: more than 99% of Pb2+ is S-bound. This is very different if the coordinating properties of uridylyl-(5′→3′)-[5′]-uridylate (pUpU3−) and P-thiouridylyl-(5′→3′)-[5′]-uridylate (pUp(S)U3−) are compared. The phosphate-coordinated Pb2+ forms a 10-membered chelate with one of the two terminal O atoms of the phosphodiester linkage, which reaches a formation degree of about 90% in Pb(pUpU)−. However, in Pb(pUp(S)U)− the formation degree of the chelate is reduced to about half in accordance with the fact that now only one terminal O atom is available in the thiophosphate diester bridge, that is, Pb2+ coordinates to this O showing no affinity for S in ROP(O)(S)−OR′. These observations are ascribed to the properties of the Pb2+ lone pair, which shapes the Pb2+ coordination sphere; its role is discussed further in this Perspective and a caveat is made regarding Pb2+ binding to a thiophosphate diester linkage.
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...