Oxone-mediated annulation of 2-aminobenzamides and 1,2-diaminobenzenes with sec-amines via imine-N-oxides: new syntheses of 2,3-dihydroquinazolin-4(1H)-ones and 1H-benzimidazoles†
Abstract
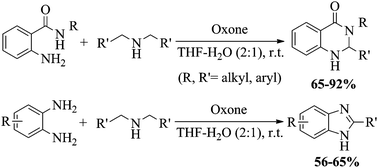
An efficient and mild method for the preparation of 2,3-dihydroquinazolin-4(1H)-ones and 1H-benzimidazoles by the oxone-mediated reaction of sec-amines via imine-N-oxides with 2-amino-N-substituted benzamides and 1,2-diaminobenzenes respectively in THF–water (2 : 1) at ambient temperature is described.


 Please wait while we load your content...
Please wait while we load your content...