New insights into the protogenic and spectroscopic properties of commercial tannic acid: the role of gallic acid impurities†
Abstract
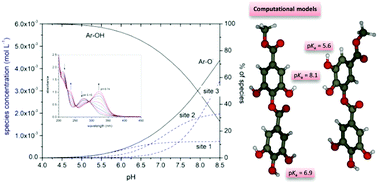
Tannic acid (TA) belongs to the class of hydrolysable tannins that are natural polymers derived from the vegetable kingdom. Although TA is described as a molecule with a central core of glucose esterified with five digallic units, its molecular structure has not yet been completely clarified. Actually, TA is described as a mixture of different compounds. In this work, by using potentiometry, UV-vis and fluorescence spectroscopy, as well as ab initio calculations we identified the protonation properties and spectroscopic features of TA. A preliminary investigation on gallic acid (GA), present as an impurity in the commercial TA mixture, and on methyl 3,4,5-trihydroxybenzoate served as a benchmark for the computational work and gave insight into the contribution of GA to the TA properties. GA principally affects the pH of TA solutions and the fluorescence signals. The data showed the presence of three main types of protogenic groups that can be assigned to the typical TA structure, with pKa values included in the range of 6–8.5, which can be ascribed to the phenolic functions. The least acidic site shows the highest concentrations, and the dissociation of half of the TA phenolic groups takes place at pH ∼ 7.8. The UV-vis spectra of the protonated and deprotonated species were obtained through data elaboration by stoichiometric and chemometric approaches. The results show main absorption maxima (277 and 323 nm, respectively) similar to those obtained using ab initio calculations. Overall, we achieved a remarkable coherence among the outcomes obtained by using different methodologies.
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...