Thermodynamic study on 8-hydroxyquinoline-2-carboxylic acid as a chelating agent for iron found in the gut of Noctuid larvae†
Abstract
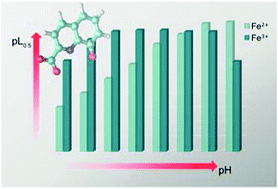
The recognition of quinolinic carboxylic acids as natural chelants and the recent observation of a high production of 8-hydroxyquinoline-2-carboxylic acid (8-HQA) in the gut of several Noctuid larvae (e.g. Spodoptera littoralis) has inspired the study of the chelation properties of 8-HQA towards Fe2+ and Fe3+. Here, we report a detailed characterization of the thermodynamic solution behaviour of Fe2+/8-HQA and Fe3+/8-HQA systems as a function of the pH value. The acid–base properties of 8-HQA and its binding ability towards Fe2+ and Fe3+ have been investigated over a wide range of pH values (2.0 ≤ pH ≤ 11.0) by ISE-H+ (glass electrode) potentiometric titrations in KCl(aq) at I = 0.2 mol dm−3 and at T = 298.15 K. For both oxidation states, various FeLqHr species are formed, with q = 1, 2 (and 3), and −2 ≤ r ≤ 1. The presence of the main FeLqHr species was confirmed by HESI-HRMS. ESR measurements have also been performed to get some extra information on the Fe3+ coordination, indicating a distorted octahedral symmetry around the metal center. Quantum mechanical calculations have been carried out in order to characterize the structural features of selected metal complexes. The complexing ability of 8-HQA is generally much higher for Fe3+ than Fe2+. Nevertheless, the sequestering ability of 8-HQA towards these two oxidation states of this metal ion, obtained by the calculation of several pL0.5 values, resulted in it being highly dependent on the pH value: (i) at relatively low pH values, it is higher for Fe3+ (pL0.5 = 6.3 at pH = 3.0) than for Fe2+ (pL0.5 = 3.1 at pH = 3.0); (ii) it is almost the same at pH = 8.1 (Fe3+: pL0.5 = 8.3; Fe2+: pL0.5 = 8.1); (iii) it is higher for Fe2+ at high pH values (pL0.5 = 8.9 for Fe2+ and pL0.5 = 6.2 for Fe3+ at pH = 10.0). The determination of the stability constants of the Fe2+/8-HQA and Fe3+/8-HQA complexes was also complemented by data obtained by the ligand-competition approach, using EDTA as a competing ligand over a wide range of cation and ligand concentrations and ratios. This also allowed a more thorough investigation of both the Fe2+/EDTA and Fe3+/EDTA systems, providing an accurate stability constant dataset for the Fep(EDTA)qHr species under the above-mentioned experimental conditions, which are commonly used in biological studies.
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...