Solvent effect on a model SNAr reaction in ionic liquid/water mixtures at different compositions†
Abstract
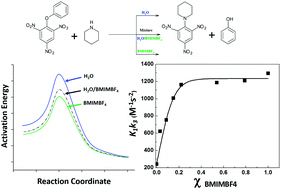
The reaction of phenyl 2,4,6-trinitrophenyl ether and piperidine was kinetically evaluated in BMIMBF4/water mixtures as the reaction media. This study shows the dramatic effect of the mixture composition on the reacting pair and its reaction rate, highlighting two strongly demarcated zones. The first one, rich in water, is characterized by strong variations in the rate coefficient values, suggesting the presence of preferential solvent effects in the aqueous phase. The second zone shows high rate coefficient values independent of the composition of the solvent mixture, suggesting predominant “anion” solvent effects. These results were validated using fluorescence spectroscopy and the Kamlet–Taft parameter.


 Please wait while we load your content...
Please wait while we load your content...