On the copper(ii) binding of asymmetrically functionalized tripodal peptides: solution equilibrium, structure, and enzyme mimicking†
Abstract
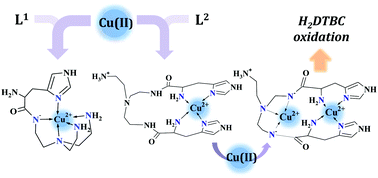
Our aim is to combine the preorganized structure of tripodal scaffolds and the advantageous metal-binding ability of histidine subunits. To this end, recently we have studied the copper(II) complexes of the tris(L-histidyl)-functionalized tren derivative, tren3his. Here we report the copper(II)-binding properties of the mono- and bis(L-histidyl)-functionalized tren ligands (tren1his (L1) and tren2his (L2)), and thus explore the impact of increasing histidine ‘density’ on the copper(II) binding of these tripodal peptides. Our solution equilibrium study was supplemented by several (UV-vis, CD, ESR, and NMR) spectroscopic and MS methods. The mono-His derivative L1 forms only mononuclear complexes. Above pH 4, the tren-like subunit is the main binding site, which is supplemented by an imidazole coordination. In the case of L2, both mono- and dinuclear copper(II) species are formed. In the acidic–neutral pH range, the highly stable bis-histamine-type binding mode dominates in the equimolar solution, while at a higher pH amide coordinated complexes are present. The bis-histamine coordination in CuHL2 creates a preorganized structure, which promotes the binding of a second metal at the tren-like binding site with {N−,Ntert,N−} coordination in Cu2H−1L2/Cu2H−2L2. Only the dinuclear Cu2H−2L2 complex was found to efficiently catalyze the oxidation of H2DTBC. The kinetic data resulted in a very high kcat/KM ratio (4360 M−1 s−1), which is due to the exceptionally strong substrate-binding ability of the dinuclear complex. However, the oxidation of the substrate within this adduct, which is a common feature of catalytically active dicopper(II) complexes, does not occur in the absence of dioxygen. This finding implies that the role of dioxygen is the oxidation of the substrate activated by the dinuclear complex. We assume a Cu(II)Cu(II)–catecholate–Cu(II)Cu(I)–semiquinone valence tautomer (VT) equilibrium. The semiquinone formed in small quantities reacts with dioxygen in a rate-determining step, which eventually results in DTBQ and H2O2 products. The Cu(II)–L2 complexes also exhibit efficient superoxide dismutase-like activity, supporting the versatility of tripodal peptide complexes in redox enzyme mimicking.
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...