Complexation of protactinium(v) with nitrilotriacetic acid: a study at the tracer scale†
Abstract
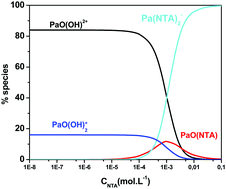
Complexation of Pa(V) with nitrilotriacetic acid (NTA) in aqueous solution (1 M (Na,H)ClO4) was studied by solvent extraction at different acidities (pcH = 0.6; 1.0; 2.0 and 2.5) with the element at the tracer scale (CPa < 10−10 M). Two types of metal–ligand complexes were identified in solution: (1 : 1) and (1 : 2) complexes. The mean charge of the two successive complexes formed in aqueous phase was deduced from the variations of Pa distribution coefficient as a function of acidity. The apparent formation constants of PaO(NTA) and PaO(NTA)23− were also determined from the solvent extraction experiments. Capillary electrophoresis coupled with inductively coupled plasma mass spectrometry (CE-ICP-MS) experiments confirmed the charge −3 of the complex of maximum stoichiometry.
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...