Hydrogen-bonding cyclodiphosphazanes: superior effects of 3,5-(CF3)2-substitution in anion-recognition and counter-ion catalysis†
Abstract
New HB-cyclodiphosph(V)azanes with a variety of structural modifications, e.g. unsymmetrical substitution of phosphorus atoms with sulfur and oxygen atoms as well as either phenyl- (O(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)/S(
P)/S(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)-13) or 3,5-(CF3)2-C6H3-substitution (O(
P)-13) or 3,5-(CF3)2-C6H3-substitution (O(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)/S(
P)/S(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)-14) and 3,5-F2C6H3-substituted cyclodiphosph(V)azanes with either oxygen (O(
P)-14) and 3,5-F2C6H3-substituted cyclodiphosph(V)azanes with either oxygen (O(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)-15) or sulfur (S(
P)-15) or sulfur (S(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)-16) substitution at the phosphorus atoms, are synthesized. These new systems are employed together with sulfur substituted cyclodiphosph(V)azanes with phenyl- (11) and 3,5-(CF3)2-C6H3-substitution (12) in recognitions of chloride and acetate anions. These HB-systems are compared to the previously established reference systems, i.e. cyclodiphosph(V)azanes (4, 5), thiourea (20) and squaramides (21, 22). Modifications of the chalcogen atom in the cyclodiphosph(V)azane moieties from oxygen (O(
P)-16) substitution at the phosphorus atoms, are synthesized. These new systems are employed together with sulfur substituted cyclodiphosph(V)azanes with phenyl- (11) and 3,5-(CF3)2-C6H3-substitution (12) in recognitions of chloride and acetate anions. These HB-systems are compared to the previously established reference systems, i.e. cyclodiphosph(V)azanes (4, 5), thiourea (20) and squaramides (21, 22). Modifications of the chalcogen atom in the cyclodiphosph(V)azane moieties from oxygen (O(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)-5) to sulfur (O(
P)-5) to sulfur (O(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)/S(
P)/S(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)-14, S(
P)-14, S(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)-12) reveal a decrease in anion binding capabilities. 3,5-(CF3)2-C6H3 substituted O(
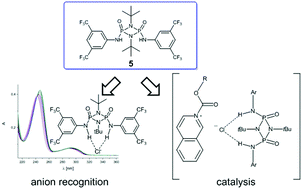
P)-12) reveal a decrease in anion binding capabilities. 3,5-(CF3)2-C6H3 substituted O(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)-cyclodiphosph(V)azane 5 exhibits the strongest anion binding effect (chloride: log[K] 5.91, log[K] acetate: 6.06) in acetonitrile, surpassing even the established thiourea 20 (chloride: log[K] 4.30, log[K] acetate: 5.47) as well as squaramides 21 (chloride: log[K] 4.92, acetate: log[K] 4.24) and 22 (chloride: log[K] 5.13, acetate: log[K] 5.37). Computational studies confirm 3,5-(CF3)2-C6H3 substituted 5 to be the strongest here studied anion-binding cyclodiphosph(V)azane with computed binding energies ΔGin–out·Cl of −21.1 kcal mol−1 and ΔGin–out·OAc of −14.3 kcal mol−1, surpassing thiourea 20 (ΔGin–out·Cl = −19.10 kcal mol−1, ΔGin–out·OAc = −13.81 kcal mol−1). The catalytic efficiency of 3,5-(CF3)2-C6H3 substituted cyclodiphosph(V)azane 5 is examined in a N-acyl-Mannich reaction, showing a significantly higher reactivity (up to 45% yield) compared to the alternative hydrogen-bonding catalyst di(1-naphthyl)silanediol 28. In all these applications, the superiority of the 3,5-(CF3)2-C6H3 substitution pattern in combination with O(
P)-cyclodiphosph(V)azane 5 exhibits the strongest anion binding effect (chloride: log[K] 5.91, log[K] acetate: 6.06) in acetonitrile, surpassing even the established thiourea 20 (chloride: log[K] 4.30, log[K] acetate: 5.47) as well as squaramides 21 (chloride: log[K] 4.92, acetate: log[K] 4.24) and 22 (chloride: log[K] 5.13, acetate: log[K] 5.37). Computational studies confirm 3,5-(CF3)2-C6H3 substituted 5 to be the strongest here studied anion-binding cyclodiphosph(V)azane with computed binding energies ΔGin–out·Cl of −21.1 kcal mol−1 and ΔGin–out·OAc of −14.3 kcal mol−1, surpassing thiourea 20 (ΔGin–out·Cl = −19.10 kcal mol−1, ΔGin–out·OAc = −13.81 kcal mol−1). The catalytic efficiency of 3,5-(CF3)2-C6H3 substituted cyclodiphosph(V)azane 5 is examined in a N-acyl-Mannich reaction, showing a significantly higher reactivity (up to 45% yield) compared to the alternative hydrogen-bonding catalyst di(1-naphthyl)silanediol 28. In all these applications, the superiority of the 3,5-(CF3)2-C6H3 substitution pattern in combination with O(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)-groups in the cyclodiphosph(V)azane scaffold is apparent.
P)-groups in the cyclodiphosph(V)azane scaffold is apparent.


 Please wait while we load your content...
Please wait while we load your content...