Phytate–molybdate(vi) interactions in NaCl(aq) at different ionic strengths: unusual behaviour of the protonated species†
Abstract
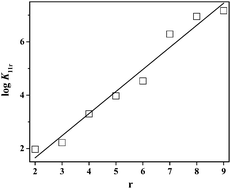
The complex formation between molybdate(VI) (MoO42−) and phytate [Phy, 1,2,3,4,5,6-hexakis(dihydrogen phosphate)myo-inositol] has been investigated at T = 298.15 K in NaCl(aq) at different ionic strengths (0 < I/mol dm−3 ≤ 1.0). Under these experimental conditions, eight (MoO4)(Phy)Hr protonated species are formed, with 2 ≤ r ≤ 9. The corresponding formation constants determined proved to be fairly dependent on ionic strength, and this dependence has been modelled by an extended Debye–Hückel equation. Furthermore, the stability (in terms of stepwise equilibria) of (MoO4)(Phy)Hr complexes regularly increases with the number of protons in the species, probably due to the formation of intermolecular hydrogen bonds between molybdate and phytate in their protonated forms. This unusual behaviour affects the sequestering ability of phytate towards molybdate, which is higher at low rather than at high pH, as demonstrated by the analysis of the pL0.5 values calculated for this system under various conditions. Finally, a series of empirical relationships have been proposed, with the aim of defining the speciation and the sequestration of molybdate and phytate in a wide number of systems.
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...