Eight homodinuclear lanthanide complexes prepared from a quinoline based ligand: structural diversity and single-molecule magnetism behaviour†
Abstract
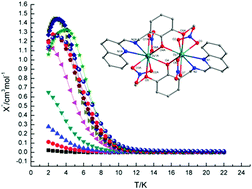
Eight dinuclear lanthanide complexes of compositions [Pr2L2(NO3)4(H2O)]·EtOH (1), [Nd2L2(NO3)4(CH3CN)]·CH3CN (2), [Sm2L2(NO3)4(CH3CN)]·CH3CN (3), [Eu2L2(NO3)4(H2O)] (4), [Tb2L2(NO3)4] (5), [Dy2L2(NO3)4] (6), [Ho2L2(NO3)4] (7) and [Er2L2(NO3)4] (8) (HL = 2-{[(2-hydroxy-3-methoxybenzyl)imino]methyl}quinoline-2-formaldehyde) have been synthesized by reactions of Ln(NO3)3·6H2O with a tetradentate quinoline-based Schiff base ligand (HL) in MeCN/EtOH/NEt3 solution. The structures of 1–8 are characterized by X-ray single crystal diffraction analysis. They exhibit four distinct structures. In complex 1, two PrIII atoms are triply bridged by three oxygen atoms, including an unusual water bridge. Complexes 2 and 3 are isostructural, in which two metal centres are nine- and ten-coordinated, respectively. There are two characteristic EuIII ions in 4, which are eight- and ten-coordinated, respectively. In the isomorphous compounds 5–8, the two LnIII atoms in the centrosymmetric molecules are doubly bridged by phenolate oxygen atoms and both metal ions possess nine-coordinate environments. Dc magnetic susceptibility studies in the 2–300 K range were performed for compounds 6 and 7. Antiferromagnetic interactions were determined for these two complexes. Complex 6 shows slow magnetic relaxation behaviour with an energy barrier (Ueff/KB) of 14.3 K.


 Please wait while we load your content...
Please wait while we load your content...