Complex formation of nickel(ii) and zinc(ii) ions with peptide fragments of rat amylin†
Abstract
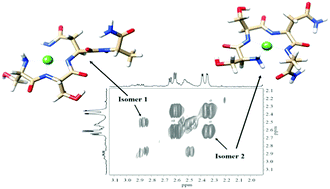
Nickel(II) and zinc(II) complexes of the 19–22 peptide fragments of rat amylin were studied by potentiometric, UV-vis, CD and NMR spectroscopic methods. The results revealed that in contrast with the corresponding copper(II) complexes, the –SSNN– sequence (or 19–22 residues of rat amylin) cannot be the primary anchoring site for nickel(II) and zinc(II) ions. For nickel(II) containing systems, an increased stability of the corresponding complexes was, however, measured and explained by an equilibrium between the common (NH2,3N−(peptide)) and (NH2,2N−(peptide),N−(asparagine)) coordination modes in a basic solution. From the comparison of the results obtained for the copper(II), nickel(II) and zinc(II) ions, it can be unambiguously stated that the rat amylin have an outstanding affinity for copper(II) binding.
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...