Assessment of the electron donor properties of substituted phenanthroline ligands in molybdenum carbonyl complexes using molecular electrostatic potentials†
Abstract
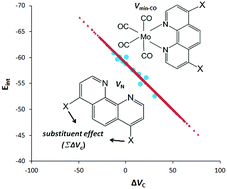
The relevance of substituent effects in the design and development of novel molecules has considerable interest in many industrial and synthetic reactions in organic chemistry. Molecular electrostatic potential (MESP) parameters are found to be excellent descriptors for characterizing the substituent and ligand effects in chemistry. Here we use MESP parameters derived for substituted benzenes as a measure of the substituent effect to assess the coordination ability of substituted 1,10-phenanthrolines with Mo(CO)4. The MESP approach used to measure the substituent effect provides an easy way to predict ligand effects before any theoretical calculations or experimental verification and it is considered to be the most accessible guideline for the quantification of the electron donating power of aromatic N-heterocyclic ligands.


 Please wait while we load your content...
Please wait while we load your content...