Molecular ordering of A(D–A′–D)2-based organic semiconductors through hydrogen bonding after simple cleavage of tert-butyloxycarbonyl protecting groups†
Abstract
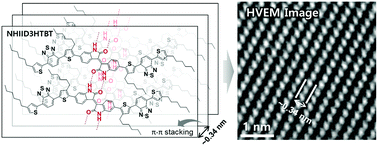
We have successfully achieved the molecular ordering of semiconducting small molecules comprising a newly designed A(D–A′–D)2 system. The A(D–A′–D)2 small molecules have two different acceptors based on isoindigo and diketopyrrolopyrrole along with tert-butoxycarbonyl (t-Boc) groups and hexyl chains, which improve the solubility of the materials. After simple thermal annealing, the t-Boc groups were removed to allow strong hydrogen bonds between N–H⋯C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O to form, and this resulted in improved molecular ordering of the organic semiconductors. The crystalline morphology was confirmed by X-ray diffraction coupled with high-voltage electron microscopy, and the resulting materials showed improved hole mobilities. In this work, the effect of the incorporation of t-Boc groups onto A(D–A′–D)2-based organic semiconductors on their morphological and electrical properties was evaluated.
O to form, and this resulted in improved molecular ordering of the organic semiconductors. The crystalline morphology was confirmed by X-ray diffraction coupled with high-voltage electron microscopy, and the resulting materials showed improved hole mobilities. In this work, the effect of the incorporation of t-Boc groups onto A(D–A′–D)2-based organic semiconductors on their morphological and electrical properties was evaluated.


 Please wait while we load your content...
Please wait while we load your content...