A novel class of photoinitiators with a thermally activated delayed fluorescence (TADF) property
Abstract
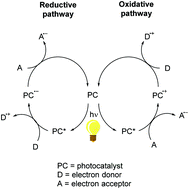
Photoinitiators exhibiting efficient thermally activated delayed fluorescence (TADF) are investigated. Known TADF metal complexes (copper-based structures) and purely organic molecules (carbazole/sulfone based organic structures) are used, for the first time, in the FRP of methacrylates, the CP of diepoxides and the synthesis of acrylate/diepoxide interpenetrated polymer networks, in thick films (1.4 mm), under air, under soft conditions using violet light delivered by a LED emitting at 405 nm. They are incorporated into two-component systems in combination with an iodonium salt and/or into three-component systems with iodonium salt/amine or N-vinylcarbazole or 9H-carbazole-9-ethanol (CARET) systems. A comparison with non-TADF analogues highlights the benefits of the TADF process. Using the copper complexes, the performances are better than those achieved with a conventional reference photoinitiator (bis acylphosphineoxide); the organic structures are noticeably less efficient. These systems exhibit a photoredox catalyst behavior. The involved chemical mechanism has been investigated using steady state photolysis, cyclic voltammetry, fluorescence spectroscopy, laser flash photolysis and electron spin resonance spin trapping techniques. The TADF property is found to be very important in increasing the excited state lifetime of the photoredox catalyst for better interactions with additives (i.e. a longer excited state lifetime is important to increase the yields of bimolecular reactions).
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...