Synthesis and investigation on optoelectronic properties of mesogenic triphenylene–perylene dyads linked by ethynylphenyl bridges†
Abstract
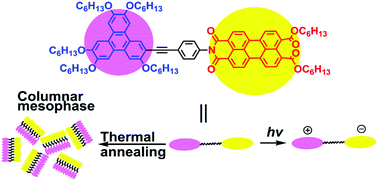
Photochemical electron donor–acceptor dyads having a penta(hexyloxy)triphenylene donor attached to the imide nitrogen atom of a perylene monoimide dihexyl ester acceptor by an ethynylphenyl bridge were prepared to give TP-n-PIE (n = 1, 2 and 3). Their molecular structures were characterized by 13C and 1H nuclear magnetic resonance (NMR) spectroscopy, infrared spectroscopy (IR), mass spectroscopy (MS) and elemental analysis (EA). Differential scanning calorimetry (DSC) traces, polarizing optical microscopy (POM) textures and X-ray diffractograms confirmed that shorter ethynylphenyl bridges facilitated the formation of a columnar hexagonal liquid crystal phase. The intensity of light absorption of the perylene units was independent of the bridge lengths, while that of the triphenylene units was red-shifted with the increase of the bridge lengths. 2D and 3D photoluminescence emission showed that the fluorescence quenching degree of perylene units increased as the rigid bridges became shorter. According to the energy level structures of the dyads, fluorescence quenching was attributed to intramolecular photoinduced electron transfer processes. And in these processes the charge-separated state of the molecule, TP+˙-1-PIE−˙, further confirmed by the photocurrent response curve, was obtained. Cyclic voltammetry revealed that although the ethynylphenyl bridges were conjugatedly connected to the triphenylene units, the highest occupied molecular orbital (HOMO) energy levels, the lowest unoccupied molecular orbital (LUMO) energy levels and the band gaps of these dyads were nearly constant when the bridge lengths varied. And these results were in agreement with those of theoretical modelling. The formation of a columnar liquid crystal phase, the large molar extinction coefficient and efficient formation of charge-separated molecules when excited give these dyads the prospect of being used as a novel type of single-component photovoltaic active materials.


 Please wait while we load your content...
Please wait while we load your content...