Dibromination of alkenes with LiBr and H2O2 under mild conditions†
Abstract
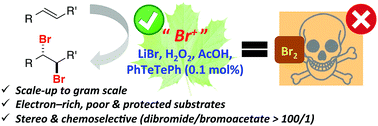
Electron-rich and electron-poor alkenes, and alkenes bearing protecting groups can be efficiently and stereoselectively converted to trans-dibromides using LiBr/H2O2 and AcOH as a proton source in 1,4-dioxane. For most substrates addition of 0.1 mol% of PhTeTePh enhances the reaction rate and the yield of the products. Experimental data suggest that the brominating agent prepared in situ is molecular bromine and that LiBr assists the activation of H2O2 allowing bromination to occur using AcOH as a mild proton source in uncatalyzed experiments. Scale-up is feasible: 10.0 mmol of 1-octene was quantitatively converted to 1,2-dibromooctene in one hour of reaction at room temperature.


 Please wait while we load your content...
Please wait while we load your content...