Design and synthesis of imidazoles linearly connected to carbocyclic and heterocyclic rings via a 1,2,3-triazole linker. Reactivity of β-azolyl enamines towards heteroaromatic azides†
Abstract
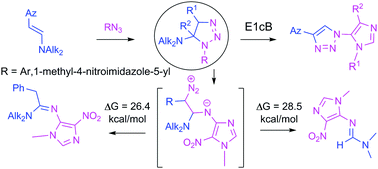
Herein we report an investigation on the 1,3-dipolar cycloaddition of 5-azido-4-nitroimidazoles to β-azolylenamines and the subsequent transformations of the intermediate 1,2,3-triazolines. 5-Azido-1-methyl-4-nitroimidazoles were combined with β-azolylenamines to form novel imidazoles linearly connected via a 1,2,3-triazole to 1,2,3-thiadiazole and isoxazole rings. On the other hand, in the reaction of β-(imidazol-4-yl)enamine with 1-methyl-4-nitro-5-azidoimidazole, the N,N-dimethyl-N′-(1-methyl-4-nitro-1H-imidazol-5-yl)formimidamide was isolated as a single product. A third type of product, active methylenes containing N-imidazol-5-yl-amidines were obtained in a similar reaction of β-phenylenamine. The factors which govern the outcome of the reactions of these heterocyclic enamines with highly electrophilic imidazolylazides were carefully studied on the basis of DFT calculations with a mPW1K density functional. The formation of various products in the reaction of imidazolyl azides with enamines was rationalized by different transformation pathways of a common 1,2,3-triazoline intermediate. According to the calculations, the two transformation paths, that are different from the path leading to aromatic 1,2,3-triazoles, occur by a stepwise mechanism involving a diazo compound formed by ring-opening of the intermediate 1,2,3-triazolines. Based on literature analysis and theoretical investigations, it is proposed that elimination of an amine from the intermediate 1,2,3-triazolines leading to 1,2,3-triazoles is a base-catalyzed process which takes place via an E1cb mechanism.


 Please wait while we load your content...
Please wait while we load your content...