Cycloplatinated(ii) complexes bearing 1,1′-bis(diphenylphosphino)ferrocene ligand: biological evaluation and molecular docking studies†
Abstract
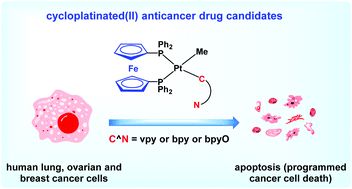
In this study, the cytotoxic activities of structurally related cycloplatinated(II) complexes containing chelating and bridging 1,1′-bis(diphenylphosphino)ferrocene (dppf) ligand derived from a wide range of C^N cyclometalating ligands (vpy = deprotonated 2-vinylpyridine, bzq = deprotonated benzo[h]quinoline, bpy = deprotonated 2,2′-bipyridine, bpyO = deprotonated 2,2′-bipyridine N-oxide, and ppy = deprotonated 2-phenylpyridine), were evaluated against human lung (A549), ovarian (SKOV3) and breast (MCF-7) cancer cell lines. The most cytotoxic compounds, 2a, 2c and 2d, effectively produced cell death by inducing apoptosis in the A549, SKOV3 and MCF-7 cancer cell lines. In addition, the molecular docking simulation was performed to determine the specific binding mode and the orientation of binding to DNA. According to the results of biological evaluation, the dppf-containing cycloplatinated(II) complexes exhibited strong interactions with DNA as well as high cytotoxicity and apoptosis-inducing activities to human cancer cell line. The present study suggests that precise rational design of new platinum-based complexes would result in the preparation of potential anticancer drugs, which can induce facile apoptosis.


 Please wait while we load your content...
Please wait while we load your content...