A simple dispersive-micro-solid phase extraction based on a colloidal silica sorbent for the spectrophotometric determination of Fe(ii) in the presence of tetrabutylammonium bromide†
Abstract
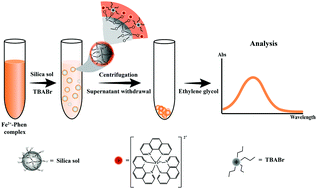
This study demonstrates a simplified dispersive micro-solid phase extraction (d-μ-SPE) using silica sol as the sorbent for the preconcentration of ferrous ions. Ferrous ions were prederivatized via complexation with 1,10-phenanthroline (Phen) before being adsorbed by the silica sol. The adsorption of the [Fe2+-Phen] complex simultaneously occurred with aggregation of the silica sol with the assistance of the phase transfer catalyst (PTC) tetrabutylammonium bromide. The enriched [Fe2+-Phen] complex on the silica sol was directly detected using a spectrophotometer at 510 nm. Several experimental parameters including the amount of silica sol, pH, PTC, extraction and centrifugation times were investigated. Under the optimal conditions, the enrichment factor was 7.3 and the linear range was from 0.07 mg L−1 to 1.43 mg L−1. The limit of detection and the limit of quantitation were 16 μg L−1 and 54 μg L−1, respectively. Furthermore, the precision, expressed as the relative standard deviation, was lower than 4% from ten replicates. The extraction recoveries of Fe2+ in cereal and vegetable samples were 71.0–107.5%. In addition, a camera in combination with Image J program was used as an alternative tool for the detection of the adsorbed [Fe2+-Phen] complex.


 Please wait while we load your content...
Please wait while we load your content...