Synthesis and structural characterization of zirconium complexes supported by tridentate pyrrolide-imino ligands with pendant N-, O- and S-donor groups and their application in ethylene polymerization†
Abstract
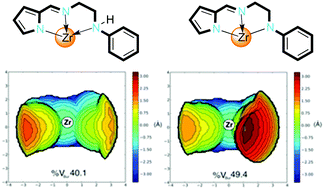
Zirconium complexes {L}ZrClx(THF)n [L = 2-(C4H3N-2′-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)C2H4NHPh, x = 3, n = 1, 2a; L = 2-(C4H3N-2′-CH
N)C2H4NHPh, x = 3, n = 1, 2a; L = 2-(C4H3N-2′-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)C6H4-2-OPh, x = 3, n = 0, 2b; L = 2-(C4H3N-2′-CH
N)C6H4-2-OPh, x = 3, n = 0, 2b; L = 2-(C4H3N-2′-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)C6H4-2-SPh, x = 3, n = 1, 2c; L = 2-(C4H3N-2′-CH
N)C6H4-2-SPh, x = 3, n = 1, 2c; L = 2-(C4H3N-2′-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)CH2C6H4-2-OMe, x = 3, n = 0, 2d; L = 2-(C4H3N-2′-CH
N)CH2C6H4-2-OMe, x = 3, n = 0, 2d; L = 2-(C4H3N-2′-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)C2H4NPh, x = 2, n = 0, 4a] were prepared and characterized by elemental analysis, NMR spectroscopy, and by X-ray crystallography for 2a and 4a. In the solid state, 2a and 4a are monomeric with the pyrrolide-imino-amine adopting a mer-configuration. Upon activation with methylaluminoxane (MAO), all Zr(IV) precatalysts were active in ethylene polymerization and exhibited high thermal stability operating effectively at 100 °C with activities up to 453 kg(PE) mol[Zr]−1 h−1. The high-density polyethylenes produced are strictly linear with melting temperatures in the range of 134–136 °C, and crystallinities varying from 56% to 70%.
N)C2H4NPh, x = 2, n = 0, 4a] were prepared and characterized by elemental analysis, NMR spectroscopy, and by X-ray crystallography for 2a and 4a. In the solid state, 2a and 4a are monomeric with the pyrrolide-imino-amine adopting a mer-configuration. Upon activation with methylaluminoxane (MAO), all Zr(IV) precatalysts were active in ethylene polymerization and exhibited high thermal stability operating effectively at 100 °C with activities up to 453 kg(PE) mol[Zr]−1 h−1. The high-density polyethylenes produced are strictly linear with melting temperatures in the range of 134–136 °C, and crystallinities varying from 56% to 70%.


 Please wait while we load your content...
Please wait while we load your content...